Figure 3. Wing coloration in Araschnia levana, with typical ‘spring form’ coloration on left and ‘summer form’ coloration on right.
I am broadly interested in the evolution and maintenance of multiple phenotypes within populations and species, including sexual dimorphism, sex-specific polymorphism and seasonal polyphenism. My research focuses on animal coloration, because these traits offer unique opportunities to probe important questions across a broad range of biological complexity, from the optical physics of color production to the interplay between sexual and natural selection at population, landscape and geographic scales.
My research addresses questions of mechanism and theory along the trajectory illustrated in Figure 1 below, in an attempt to understand both pattern and process. During my doctoral studies with Ron Rutowski at Arizona State University, I focused on the evolution of sexual dichromatism in Cabbage White butterflies (Pieris rapae). This work revealed the following narrative. Male Pieris rapae are more colorful than their female conspecifics (Figure 2). Wing coloration in this species is produced by semiordered arrays of pterin-pigment-filled nanostructures deposited in the scales covering the wings, an arrangement that simultaneously increases light reflection at long wavelengths and strongly absorbs short wavelength light. The sexual dichromatism in P. rapae is produced by increased investment of pterin pigments into male wing scales. Pterin pigments are the most nitrogen-rich pigments described from the animal kingdom, and yet larval growth in P. rapae is strongly nitrogen-limited. Females prefer to mate with more colorful males, and are able to discriminate preferred mates within natural variation in male coloration. Male coloration reflects an interaction between individual genotype and larval access to nitrogen, with the related genetic variance concentrated in the pool of genetic variation underlying larval nitrogen acquisition ability. More colorful males also have more reproductive resources to offer females during mating. Thus, sexual dichromatism in P. rapae appears to have evolved under directional selection arising from female choice. Female choice itself is likely maintained by direct and indirect benefits, both related to the higher nitrogen acquisition ability of preferred males.
As a postdoctoral researcher with Jerome Casas at the Universite de Tours, I am focused on understanding how nutritional resource dynamics have influenced the evolution of seasonal color polyphenism in the European Map butterfly (Araschnia levana). Adult A. levana are bright orange during the spring, but summer adults are black and white (Figure 3). These two seasonal color forms are produced by distinct pigment classes (ommochromes vs. melanins), which place distinct nutritional demands on developing larvae and pupae. Ommochrome synthesis relies on tryptophan as its main substrate, whereas melanins are synthesized from tyrosine. Both of these amino acid precursors are considered “essential”, that is, they must be derived directly from diet. We are studying both the pools and fluxes of these pigments and related precursors, considering the influence of diet, resource acquisiton and allocation
Figure 1. Schematic of my research program, illustrating topics addressed across a range of biological complexity.



Dr. Nathan Morehouse

2009 - Present. Postdoctoral Fellow, European Union Marie Curie International Incoming Fellowship
2009. Ph.D. Biology, Arizona State University
2000. B.S. Biology with Distinction in Research, Cornell University
Keywords:
Evolution of sexual dimorphism and polyphenism, visual ecology , evolution of sensory systems, color vision and learning, evolution of bright coloration, biophotonics, optical physics of animal coloration, pigment biochemistry, nutritional ecology and physiology, hormonal and genetic regulation of nutrient allocation, quantitative genetics, plant-herbivore co-evolution, life history evolution, sexual selection theory, selective heterogeneity at landscape and geographic scales
Sensory and nutritional ecology, evolutionary genetics

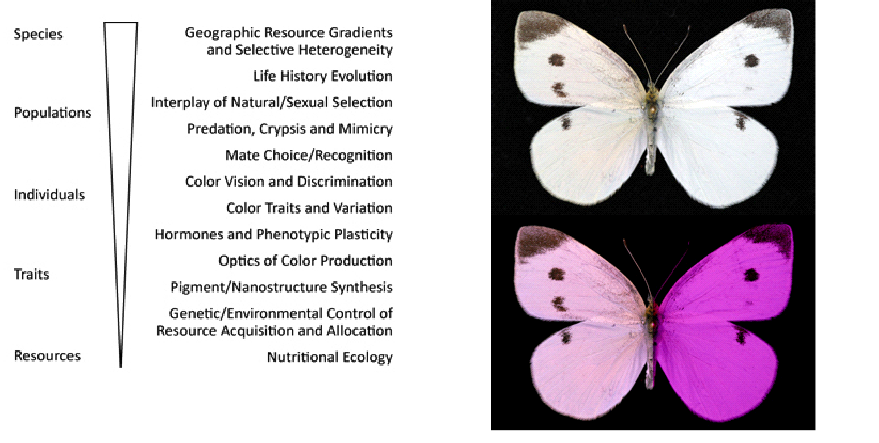

Figure 2. A Pieris rapae bilateral gynandromorph, with typical female coloration on the left and male coloration on the right (above). Below, a false-colored representation of how these wing colors might be perceived by butterfly conspecifics, which are able to see ultraviolet light.

Publication List:
Meadows, M.G., Morehouse, N.I., Rutowski, R.L, Douglas, J.M. and McGraw, K.J. Submitted. Quantifying iridescent coloration in animals: A method for improving repeatability.
Morehouse, N.I. and Rutowski, R.L. In press. In the eyes of the beholders: Female choice and avian predation risk associated with an exaggerated male butterfly color. American Naturalist.
Morehouse, N.I., Nakazawa, T., Booher, C.M., Jeyasingh, P.D. and Hall, M.D. 2010. Sex in a material world: Why the study of sexual reproduction and sex-specific traits should become more nutritionally-explicit. Oikos. doi:10.1111/j.1600-0706.2009.18569.x
Morehouse, N.I. and Rutowski, R.L. 2010. Developmental responses to variable diet composition in the cabbage white butterfly, Pieris rapae: the role of nitrogen, carbohydrates and genotype. Oikos. doi:10.1111/j.1600-0706.2009.17866.x
Van Gossum, H., Bots, J, Van Heusden, J, Hammers, M., Katleen, H. and Morehouse, N.I. 2010. Reflectance spectra and morph mating frequencies support intraspecific mimicry in the female colour polymorphic damselfly Ischnura elegans. Evolutionary Ecology.
Lindstedt, C., Morehouse, N.I., Pakkanen, H., Casas, J., Christides, J.P., Kemppainen, K., Lindström, L. and Mappes, J. 2010. Pigment composition of a variable warning signal of Parasemia plantaginis larvae (Arctiidae). Functional Ecology. doi: 10.1111/j.1365-2435.2010.01686.x
Morehouse, N.I. and Rutowski, R.L. 2009. Comment on “Floral iridescence, produced by diffraction optics, acts as a cue for animal pollinators.” Science, 325, 1072-d. doi:10.1126/science.1173324
Shawkey, M.D., Morehouse, N.I. and Vukusic, P. 2009. A protean palette: colour materials and mixing in birds and butterflies. Journal of the Royal Society Interface, 6, S221-S231. doi:10.1098/rsif.2008.0459.focus
Meadows, M.G., Butler, M.W., Morehouse, N.I., Taylor, L.A., Toomey, M.B., McGraw, K.J. and Rutowski, R.L. 2009. Iridescence: views from many angles. Journal of the Royal Society Interface, 6, S107-113. doi:10.1098/rsif.2009.0013.focus
McGraw, K.J., Toomey, M.B., Nolan, P.M., Morehouse, N.I., Massaro, M. and Jouventin, P. 2007. A description of unique fluorescent yellow pigments in penguin feathers. Pigment Cell Research, 20, 301-304. doi:10.1111/j.1600-0749.2007.00386.x
Rutowski, R.L., Macedonia, J., Merry, J., Morehouse, N.I., Yturralde, K., Taylor-Taft, L., Gaalema, D., Kemp, D.J. and Papke, R.S., 2007. Iridescent ultraviolet signaling in the Orange Sulphur butterfly (Colias eurytheme): Spatial, temporal and spectral properties. Biological Journal of the Linnean Society, 90, 349-364. doi:10.1111/j.1095-8312.2007.00749.x
Morehouse, N.I., Vukusic, P. and Rutowski, R.L. 2007. Pterin pigment granules are responsible for both broadband light scattering and wavelength selective absorption in the wing scales of pierid butterflies. Proceedings of the Royal Society of London B, 274, 359-366. doi:10.1098/rspb.2006.3730
Merry, J., Morehouse, N.I., Yturralde, K., and Rutowski, R.L., 2006. Eyes of a patrolling butterfly: Visual field and eye structure in the Orange Sulphur, Colias eurytheme (Lepidoptera, Pieridae). Journal of Insect Physiology, 52(3), 240-248. doi:10.1016/j.jinsphys.2005.11.002
Rutowski, R.L., Macedonia, J., Morehouse, N.I. and Taylor-Taft, L., 2005. Pterin pigments amplify iridescent ultraviolet signal in males of the orange sulphur butterfly, Colias eurytheme. Proceedings of the Royal Society of London B, 272, 2329-2335. doi:10.1098/rspb.2005.3216

Institut de Recherche sur la Biologie de l’Insecte
UMR CNRS 6035 UFR Sciences et Techniques
Avenue Monge - Parc Grandmont
37200 Tours
http://www.univ-tours.fr/irbi/
Phone: +33 (0)2 47 36 69 11
Fax: +33 (0)2 47 36 69 66


